The eradication of the fear of the dark (Part 1)
Over the centuries our daily life has been marked by the succession of the day-night cycle. When the night fell every activity was shut down because of the impossibility to keep it going. You may just consider that during the 18th century the only source of illumination available in towns were the torches situated near the taverns’ signs or the town gates.
The first “luminous revolution” occurred with the exploitation of fossil fuels, such as coal in the eighteenth century, and most of all the use of gas for street lighting during the first half of the 19th century.
In 1879 a second “luminous revolution” started thanks to Thomas Edison who patented the carbon filament bulb; a device 20 times more efficient than a candle (which, on the contrary, was capable of turning into light just the 0.01 % of the chemical energy of the burning wax), but still not very efficient in the conversion of electricity into light.
Filament bulb
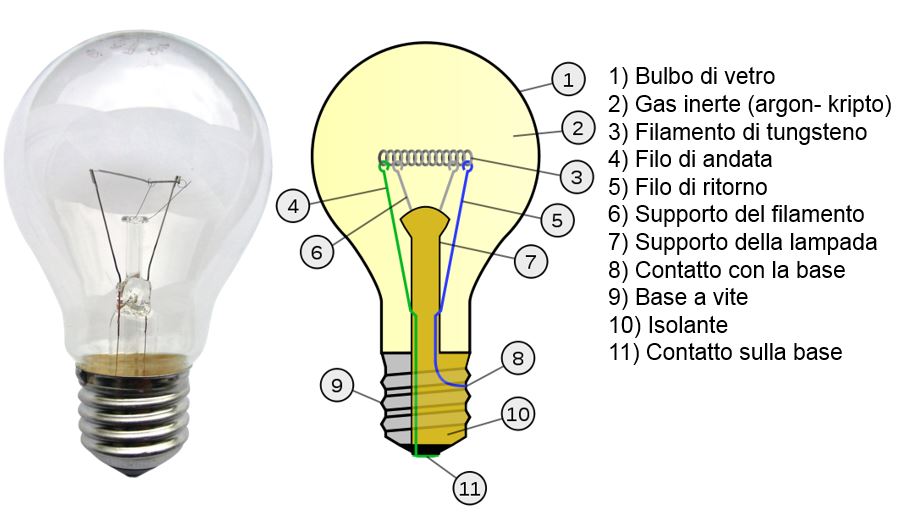
The evolution of the lamp began in 1906 with the introduction of the tungsten light bulb, in which the light is produced by the heating (on average equal to 27000 K) of a tungsten filament through which the electric current passes.
Filament lamp
The tungsten filament is heated via Joule effect (the electric power is converted in thermal energy) and brought to temperatures that causes the corrispondent blackbody spectrum to contain enough visible components to produce light. The heating increase though brings about an increase in the electric resistance and the resulting decrease in the current flowing into the bulb. The functioning is guaranteed as long as a dynamic equilibrium is established between the electric resistance and the power dispersed because of the Joule effect.

Sodium-vapor lamps
In the 1930’s the sodium-vapor lamps were introduced, with their orange-yellow colour and fluorescent tubes (improperly called neon lamps) and finally the halogen lamps in the 1960’s.
The sodium-vapor lamps are classified as “discharge lamps” and they are still available on the market in two different types:
- High-pressure sodium-vapors lamps (also known as HPS lamps)
- Low-pressure sodium-vapors lamps.
The HPS lights are mostly used in street lighting, whereas the low-pressure technology is are used in situation where a larger energy saving is needed.

Sodium-vapor lamp
Halogen lamps
Halogen lamps are a particular type of filament bulbs, among the most used in recent years until the advent of the LED lamps.
These lamps exploit the halogenic cycle that allows to triplicate the efficiency of filament bulbs. The gas mixture in the bulb includes the addition of a noble gas (which does not take part in the halogenic cycle) such as argon, krypton or xenon that cause the tungsten filament to reach the temperature of 3000 K. The tungsten evaporates and reacts with the gas forming the tungsten halide, that by making contact with the incandescent filament is decomposed and lays down the tungsten on the filament itself, fulfilling then the halogenic cycle that we discussed earlier.

Compact fluorescent lamps
In 1980 the so-called energy-saving light bulbs have been introduced, or, technically speaking, the compact fluorescent lamps (CFL), that offer a light conversion efficiency 50 times higher than the Edison lamp.
This technology achieves consumption savings up to 80% for every single light source, it costs more but the fluorescent lamps have a longer life (up to 8/10 years).
This type of lamp consist of a circular or molded glass tube so that they turn out to be more compact (hence their name), but besides their appearance the most visible difference between the CFL and the filament lamps is the lighting: the CFL needs more time to reach its maximum luminosity.

The inner surface of the tube is coated with fluorescent material that resembles white powder, and the vacuum is made inside the bulb. A low-pressure noble gas as argon, xenon, krypton or neon is inserted and also a small amount of mercury, which partially evaporates mingling with the gas.
Electrodes are put at the extremities of the tube, when the current stimulates the gases so that they produce ultraviolet radiation, the fluorescent material in turn produces visible radiation, that is…light.
In this conversion only a part of the energy released by the ultraviolet wave is turned into light, the remainder becomes heat that warms up the lamp.
In the next we will complete our review on the evolution of lamps, addressing specifically the LED technology, which nowadays is revolutionizing lighting and replacing with great success the previous models that we have just described. We will thoroughly discuss the concept of light efficiency and light pollution; see you soon and as it’s usually said in these cases…to be continued!
The eradication of the fear of the dark (Part 2)
Sources
- camasfriends
- backstagenews
- giovanigenitori
- Nicola Armaroli e Vincenzo Balzani, Energia per l’astronave Terra. L’era delle rinnovabili, 2017








